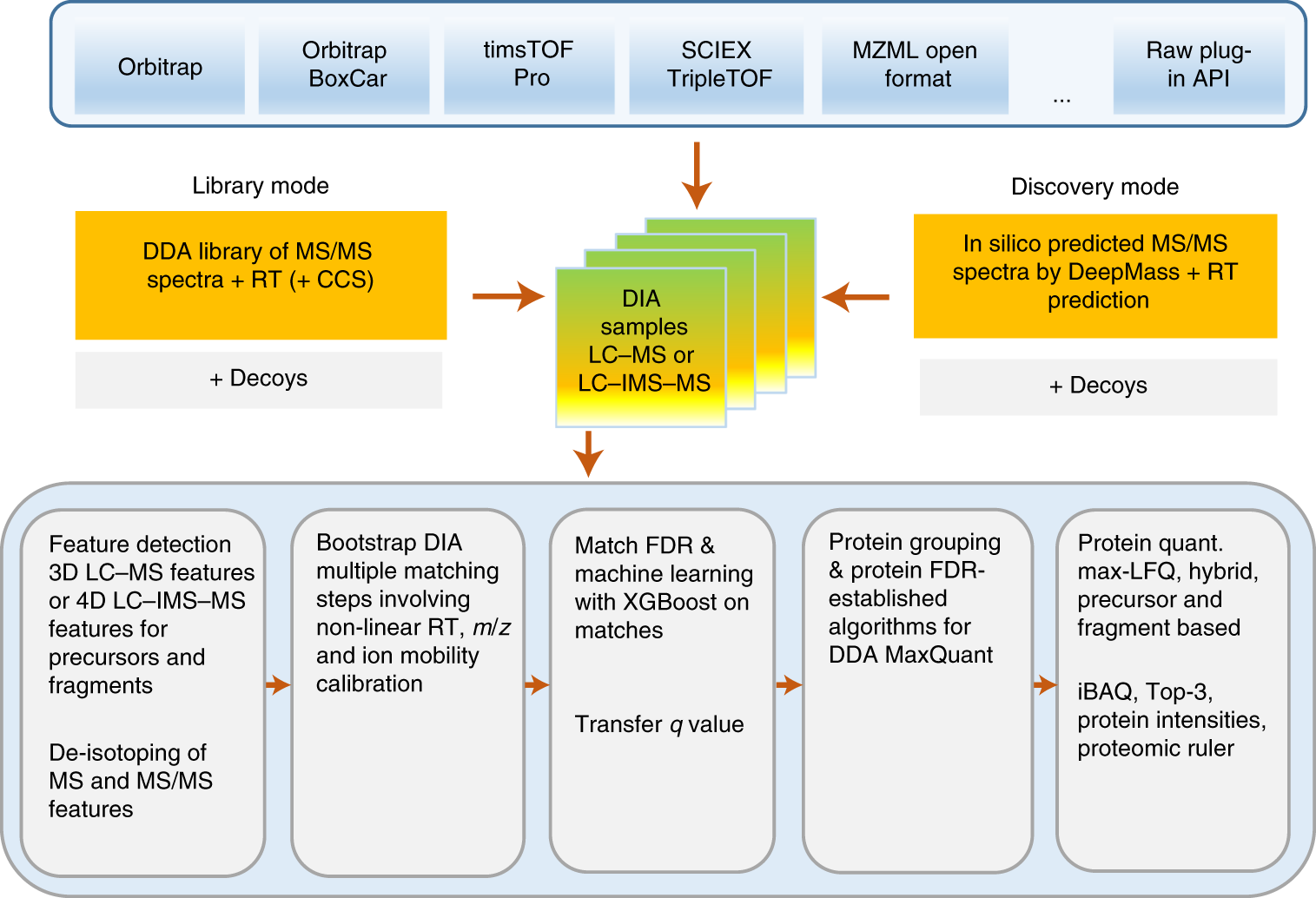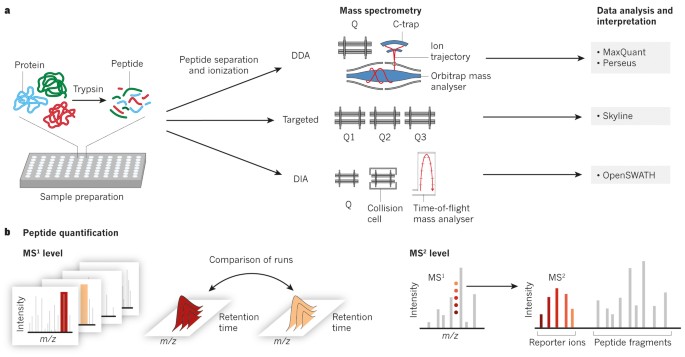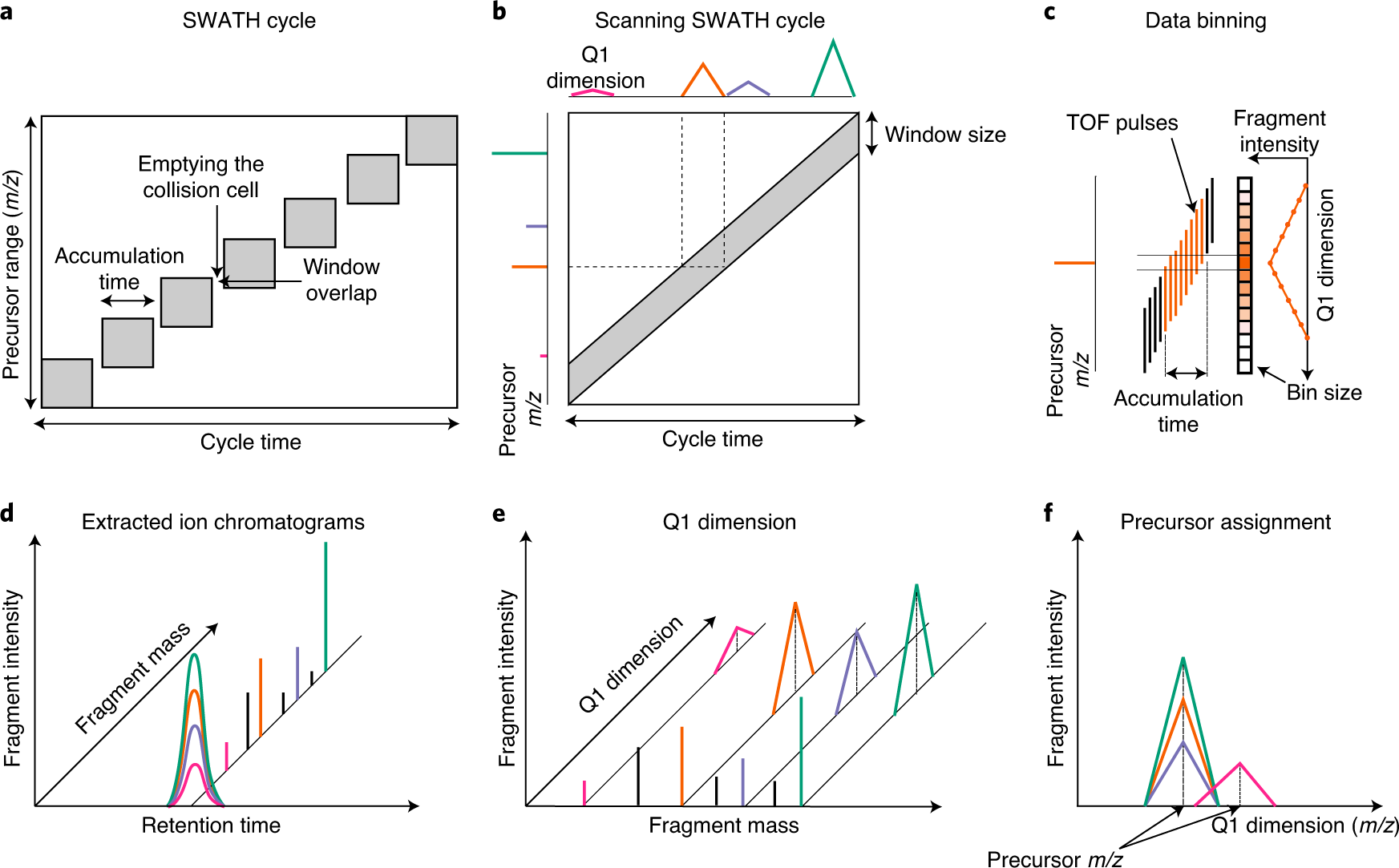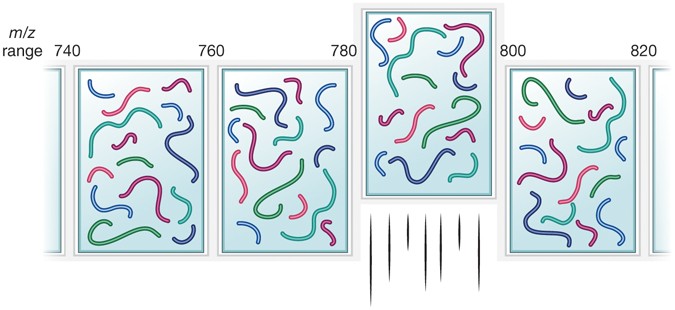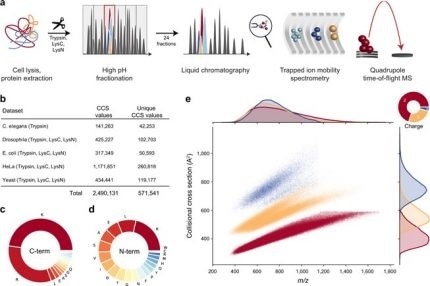
New Nature Communications publication by Mann & Theis Groups harnesses the benefits of large-scale peptide
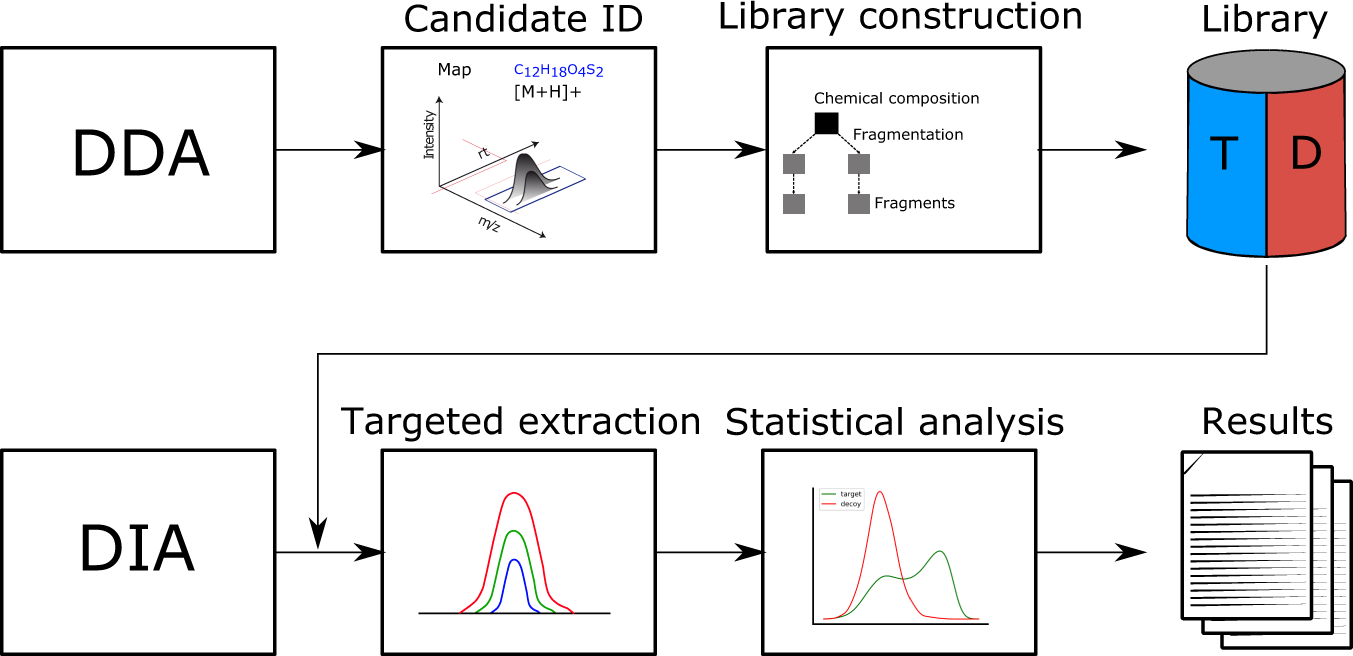
DIAMetAlyzer allows automated false-discovery rate-controlled analysis for data-independent acquisition in metabolomics | Nature Communications

Group-DIA: analyzing multiple data-independent acquisition mass spectrometry data files | Nature Methods
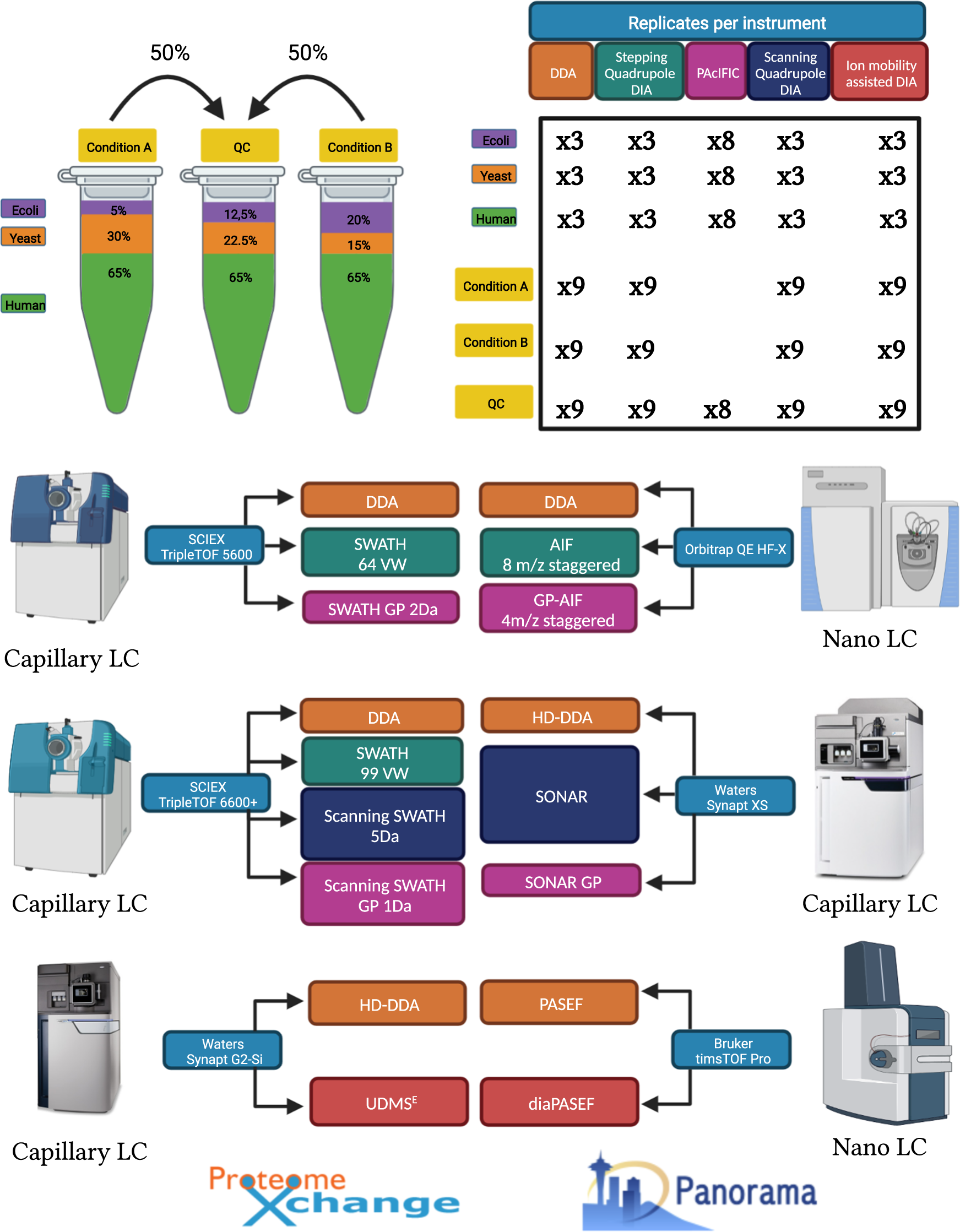
A comprehensive LFQ benchmark dataset on modern day acquisition strategies in proteomics | Scientific Data

Deep Proteomics Using Two Dimensional Data Independent Acquisition Mass Spectrometry | Analytical Chemistry
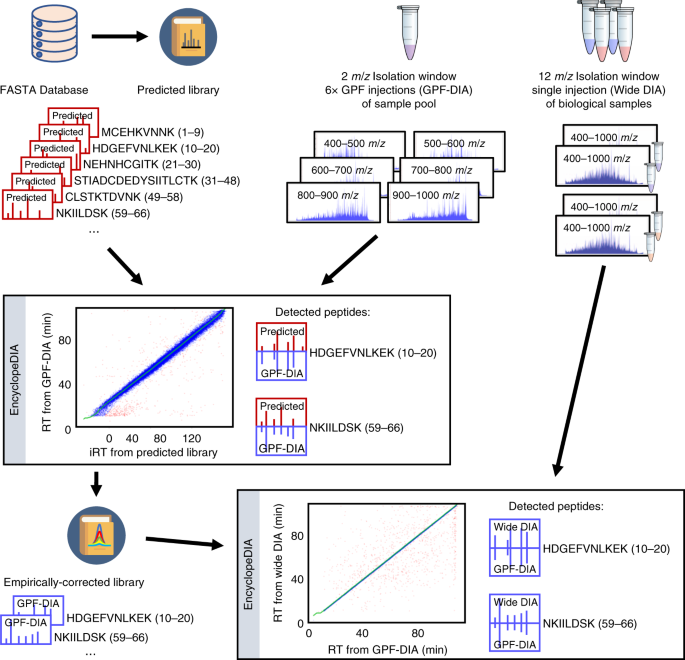
Generating high quality libraries for DIA MS with empirically corrected peptide predictions | Nature Communications

Implementing the reuse of public DIA proteomics datasets: from the PRIDE database to Expression Atlas | Scientific Data
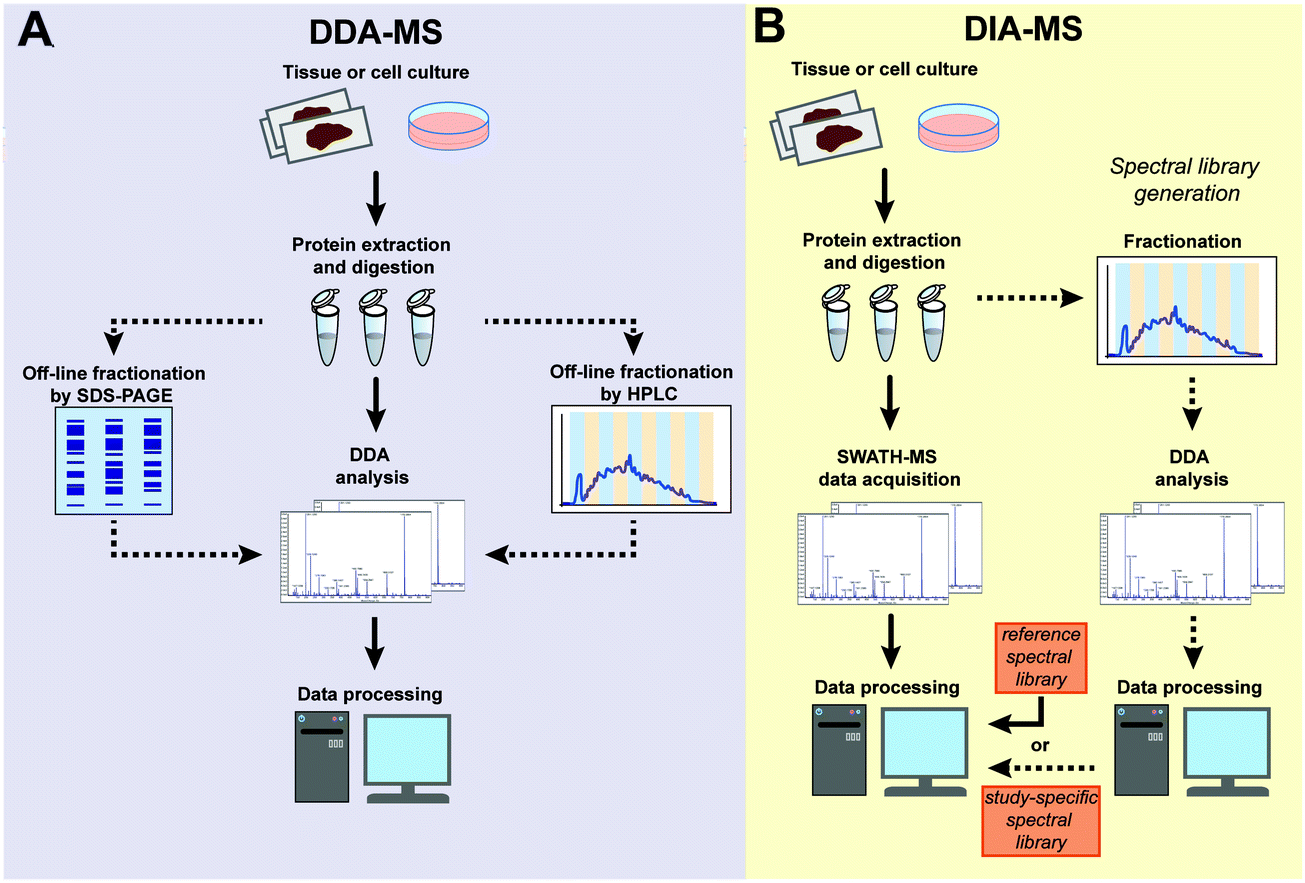
Data-independent acquisition mass spectrometry (DIA-MS) for proteomic applications in oncology - Molecular Omics (RSC Publishing) DOI:10.1039/D0MO00072H
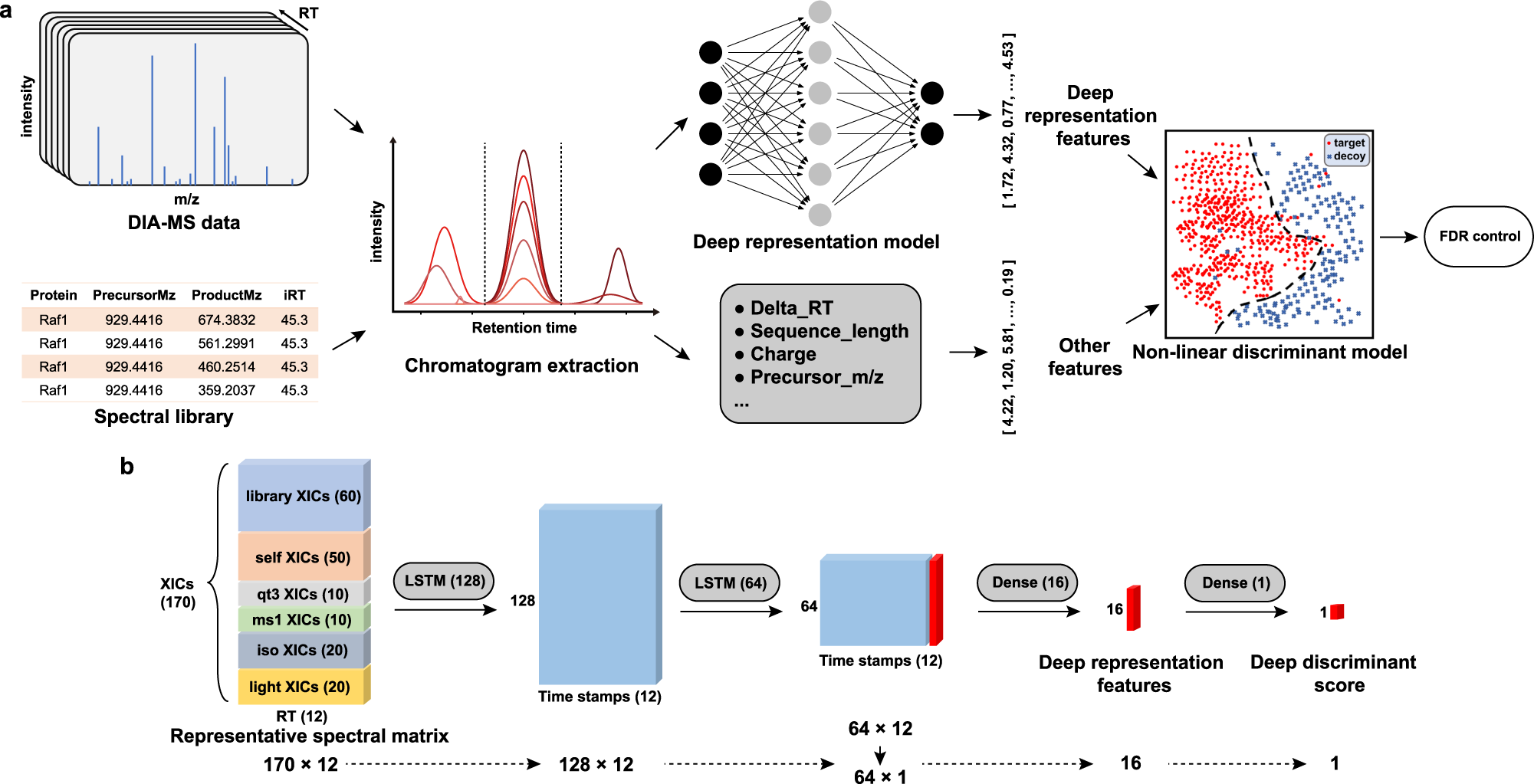
Deep representation features from DreamDIAXMBD improve the analysis of data-independent acquisition proteomics | Communications Biology
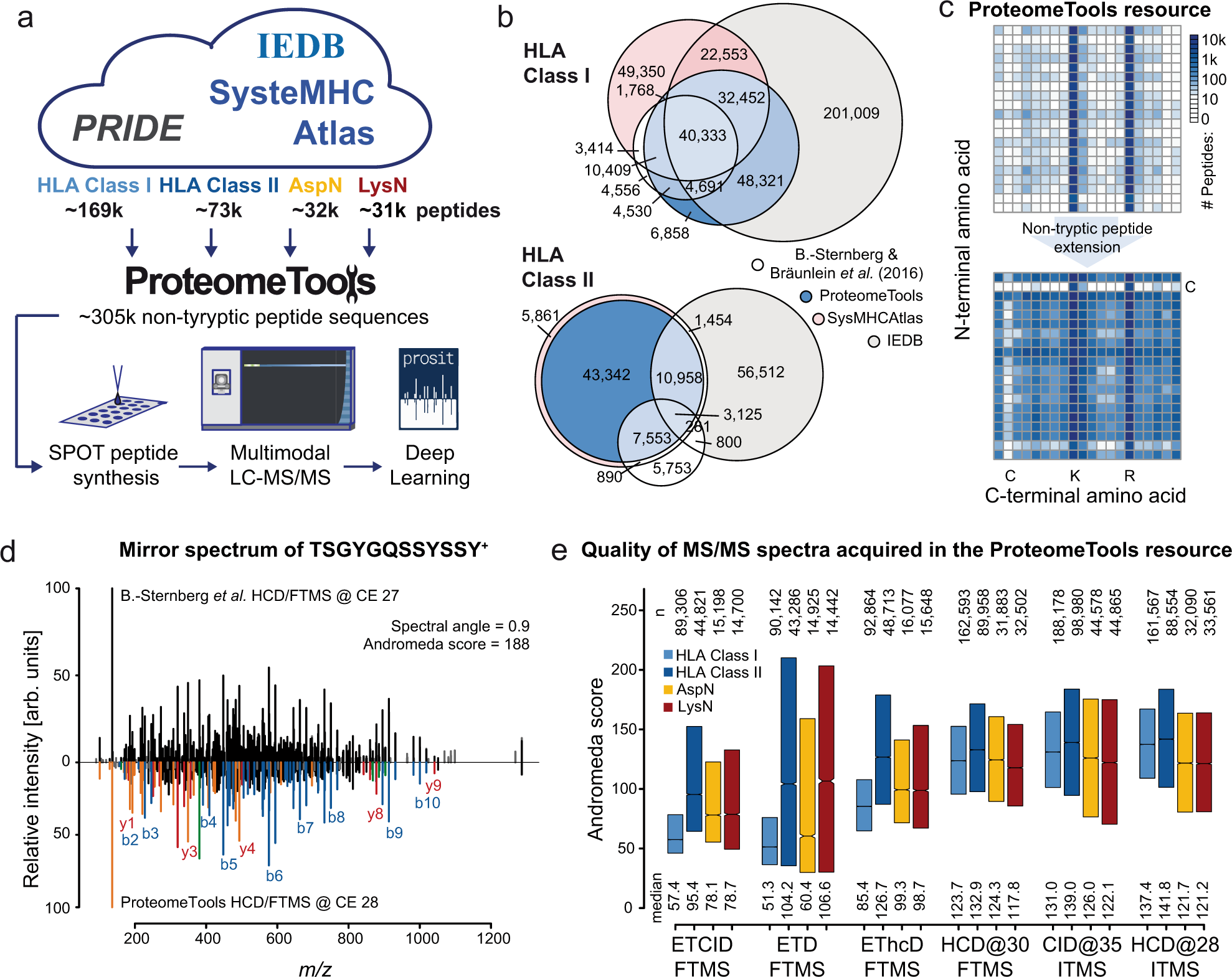
Deep learning boosts sensitivity of mass spectrometry-based immunopeptidomics | Nature Communications
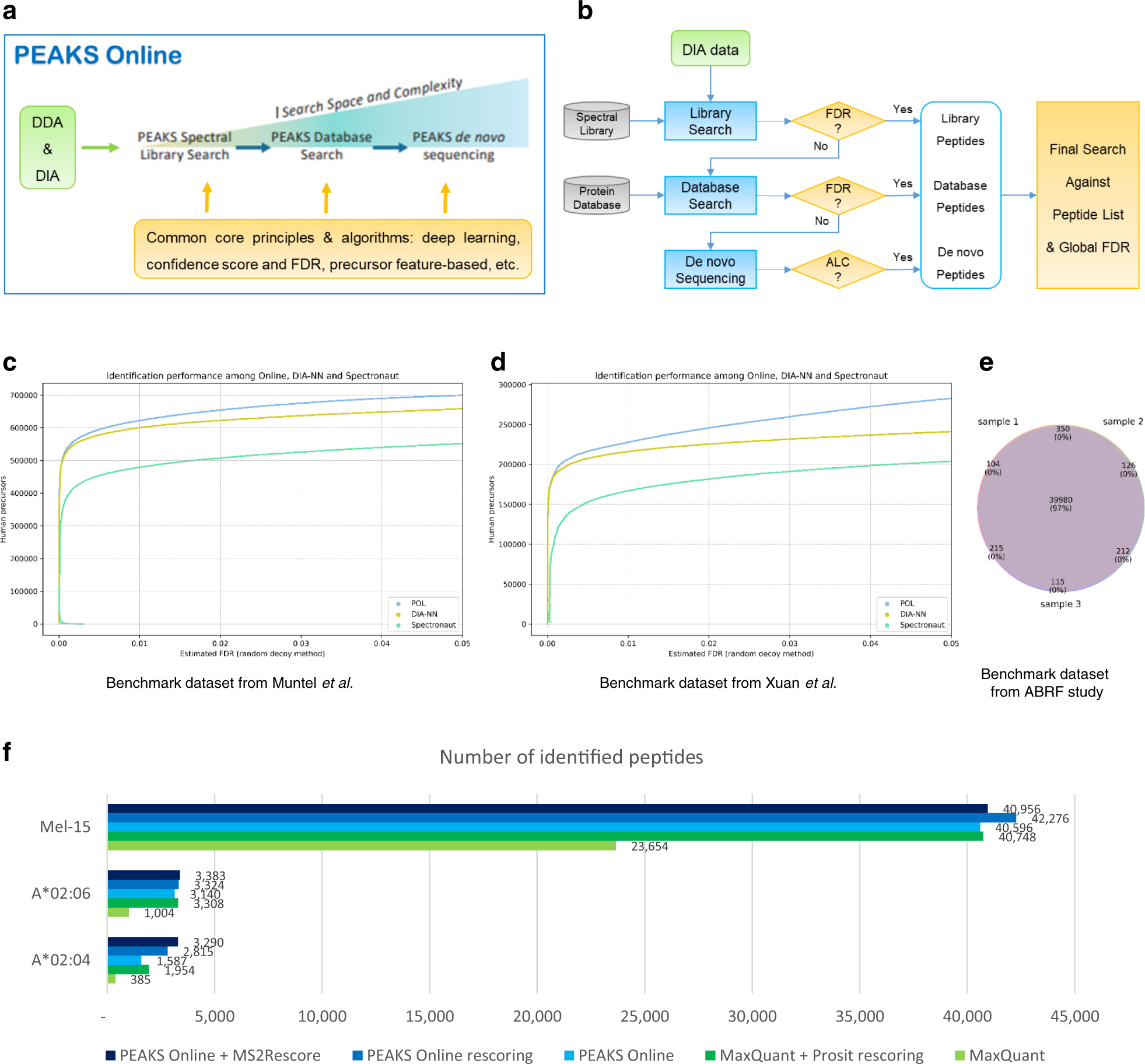
A streamlined platform for analyzing tera-scale DDA and DIA mass spectrometry data enables highly sensitive immunopeptidomics | Nature Communications

DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput | Nature Methods
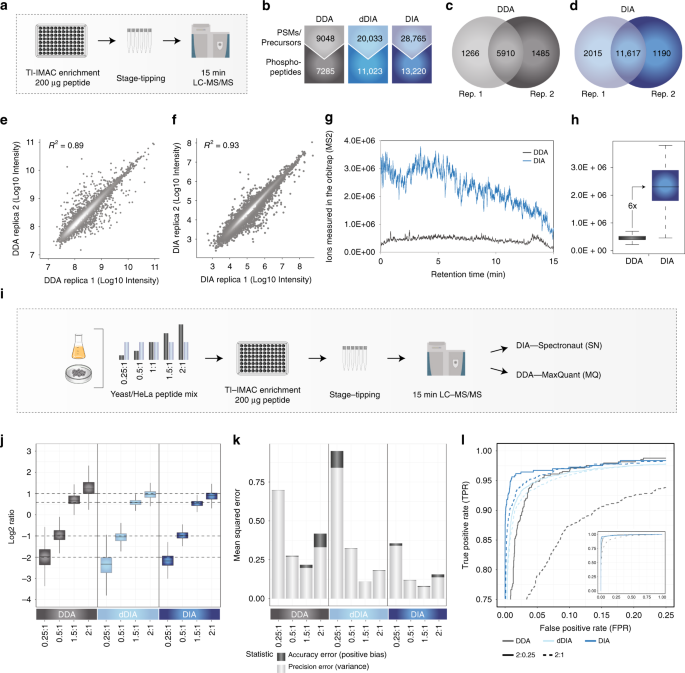
Rapid and site-specific deep phosphoproteome profiling by data-independent acquisition without the need for spectral libraries | Nature Communications
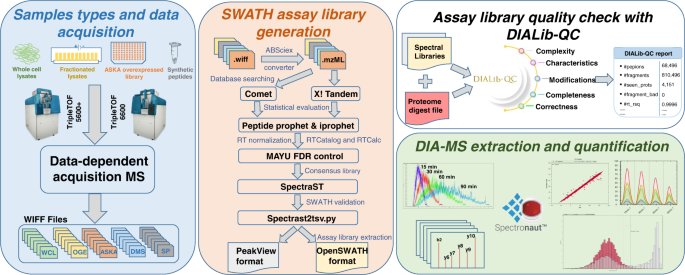
A comprehensive spectral assay library to quantify the Escherichia coli proteome by DIA/SWATH-MS | Scientific Data
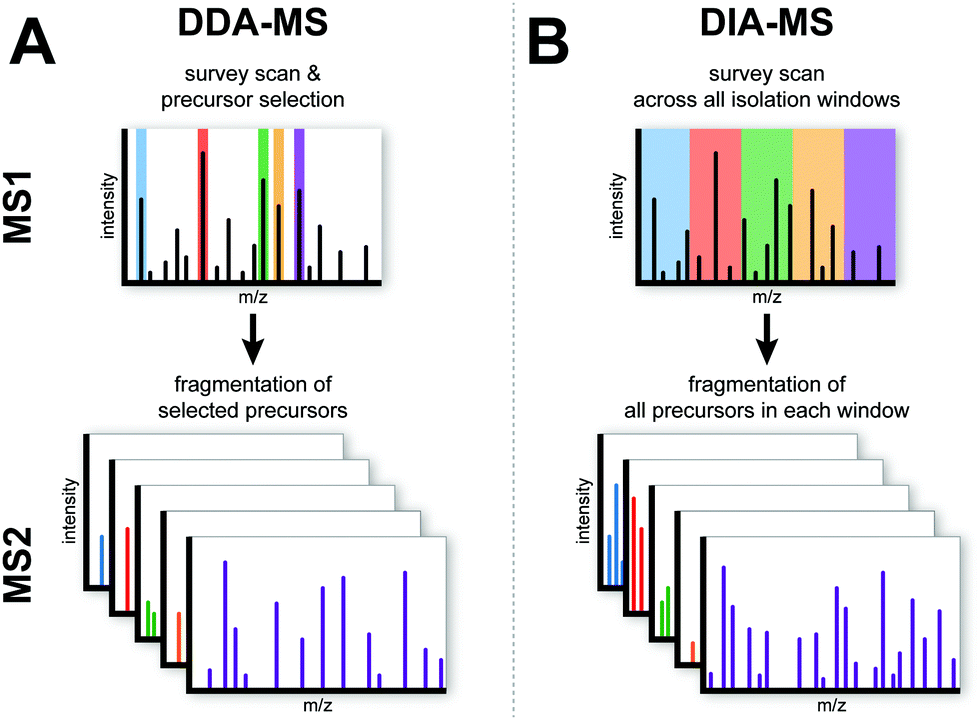
Data-independent acquisition mass spectrometry (DIA-MS) for proteomic applications in oncology - Molecular Omics (RSC Publishing) DOI:10.1039/D0MO00072H

Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry | Nature Communications
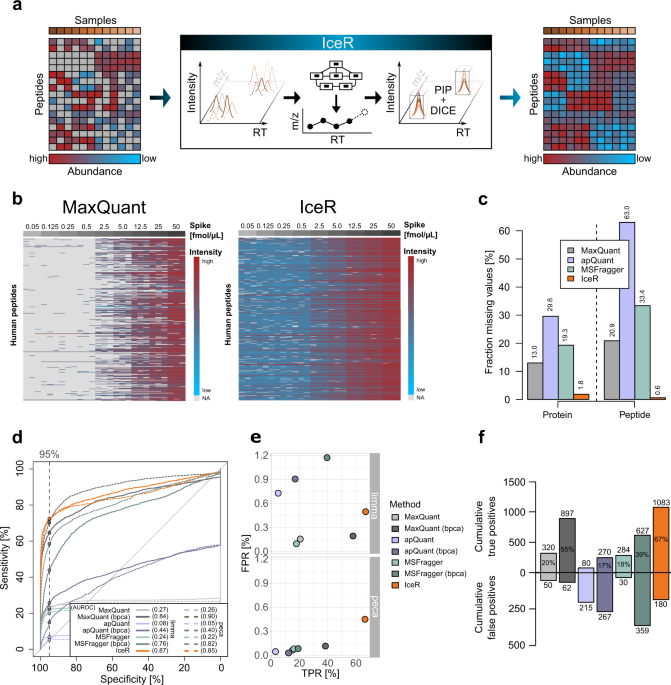
IceR improves proteome coverage and data completeness in global and single-cell proteomics | Nature Communications
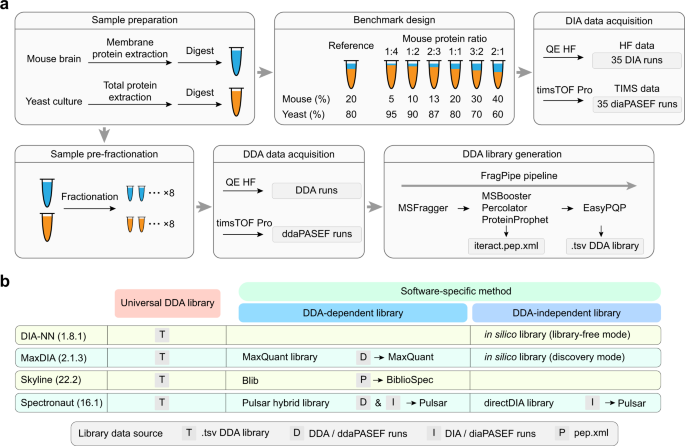
Benchmarking commonly used software suites and analysis workflows for DIA proteomics and phosphoproteomics | Nature Communications
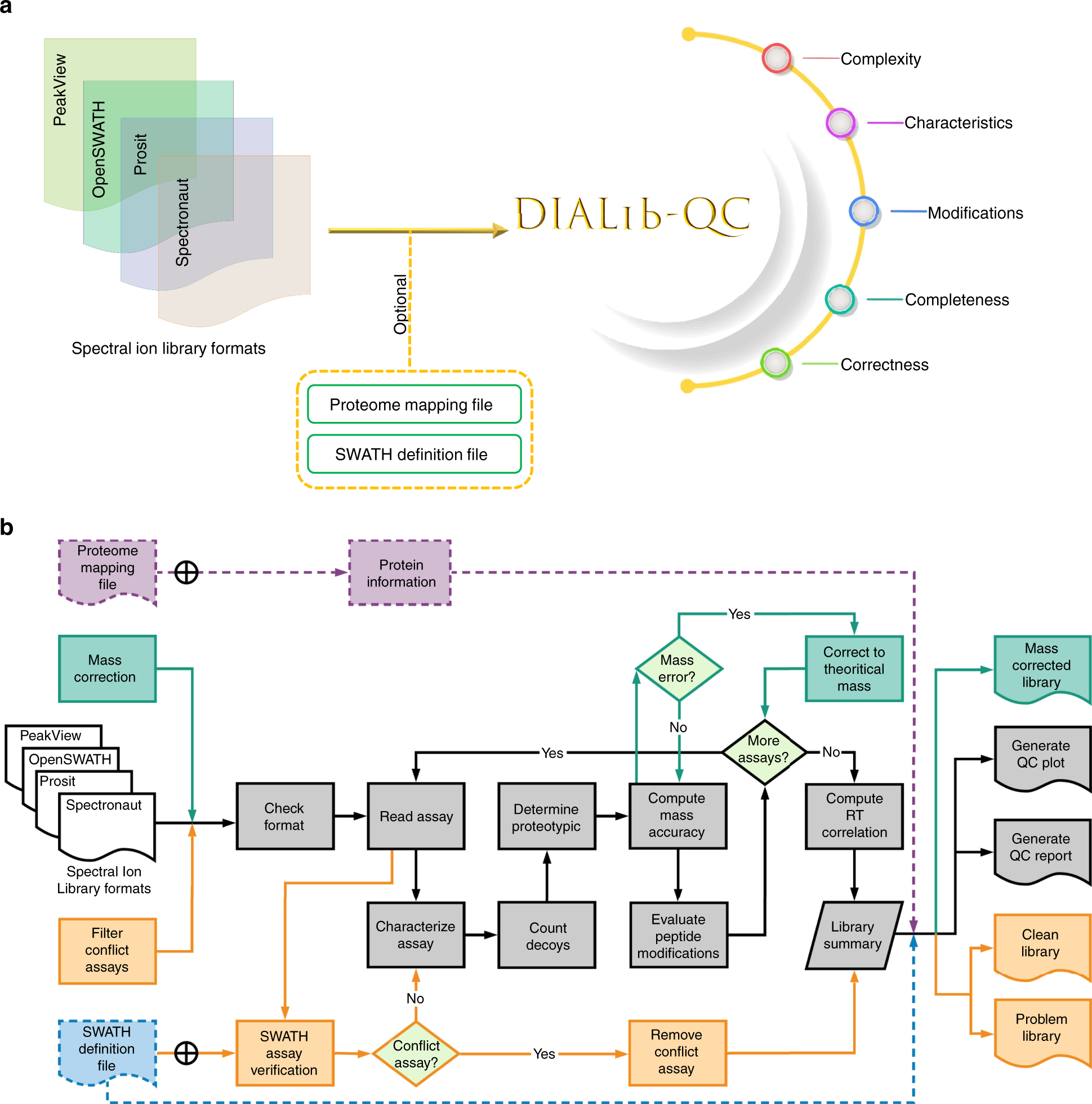
DIALib-QC an assessment tool for spectral libraries in data-independent acquisition proteomics | Nature Communications